Services
All our services are supported by our exclusive software (RIM System), which allows the safe transfer and management of data.
We offer our clients the possibility to remotely check and keep track of every project managed by MMGC.
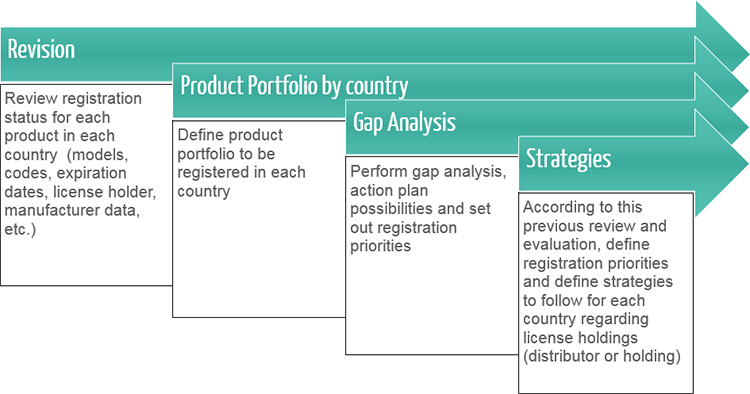
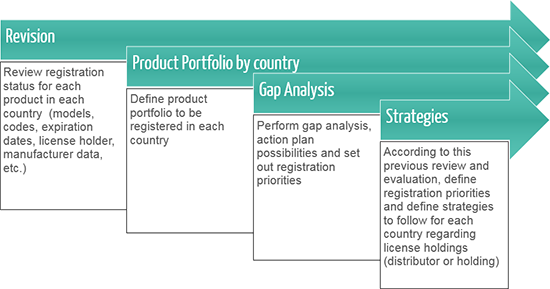
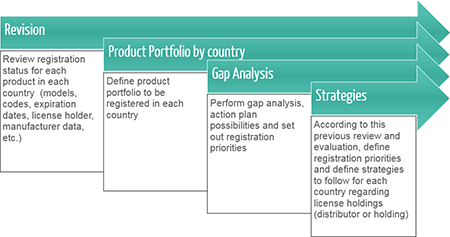
Centralized coordination and regional management of Regulatory Affairs
License Holding
In countries where the legislation allows it, we offer manufacturers the possibility to hold their registration Licenses and, in case not allowed, to have a local company not involved in the commercialization of their products keep the Licenses on their behalf and operate as their registration holder.
Registration of Medical Devices and In vitro Diagnostic medical devices
We provide assistance with the design and/or modification of medical devices to conform to the regulatory framework.
We provide support with the preparation of supporting technical documentation to obtain product approval and complying with published legislation currently in force.
We prepare national and international technical dossiers. We have extensive experience in Latin American countries, Canada, EEC.
We submit and track such documentation with the appropriate sanitary authority in Latin American countries, Canada or the United States.
Planning and Project Execution
Service for Medical Device Manufacturers and Importers.
We walk by our clients throughout the whole process from the development of their business plan, providing support and assessment to achieve all regulatory certifications applicable, until marketing and post marketing of their products in the markets of choice.
National and International Regulatory Consulting
We provide responses to specific inquiries on the regulatory matters that affect the local or global activities of your company.
Facility Design
We create layouts that are appropriate for the company’s processes, and comply with the national and international regulatory requirements.
Quality Management System
We accompany our clients in the development, implementation, integration and/or update of the Quality Management System, pursuant to ISO 13485:2016, ISO 9001:2008, ANMAT directive 3266/2013 (BPF), ANMAT directive 6052/2013 (BPADT), GMP (FDA), MDD directive 93/42/EEC.
Internal and External Auditing
We perform audits to conform to national and international standards.
In alignment with your company’s planning and priorities, we carry out audits for the purposes of diagnosing the internal systems behavior for the development of your activities and operations.
Clinical Trials
We provide a detailed checklist of requirements established by ANMAT (local Regulatory Authority) and support you throughout the whole process, from the collation and review of documentation, to the submission and approval of Clinical Trials.
Translation of Technical Documents
We translate Manuals and Instructions for Use, Technical Specifications, Clinical Trials, Validations and Test Reports, Standards and Regulations and any kind of technical-scientific documentation to support regulatory compliance.
Our translations are performed by qualified native experts with technical experience in the area, who are knowledgeable about the specific terminology of the industry of medical devices and regulatory affairs. To obtain a catalogue of these services.
Training
Based on the belief that professional qualification is an essential part of a company’s growth, we provide training and education to industry professionals.

















